Aspen API response to COVID-19 Latest update / 17 March 2022
Aspen Oss
The Netherlands
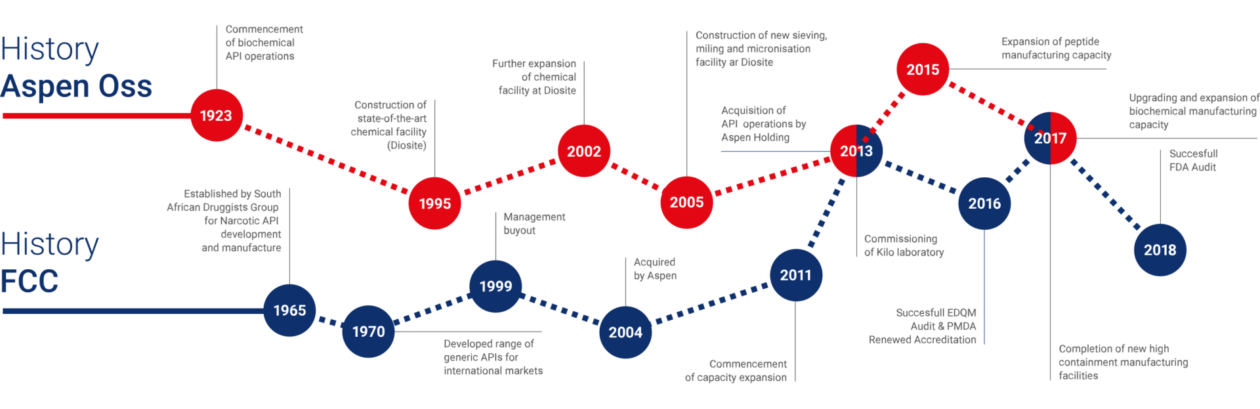
Aspen Oss has a proud history in the production of Active Pharmaceutical Ingredients, spanning over almost 100 years.
The origins date back to 1923, when the owner of a slaughterhouse initiated the extraction of animal pancreases for use in the production of insulin.
Operations have gradually expanded, culminating in Aspen Oss being recognized as a global supplier and one of the world’s best-known developers and manufacturers of steroid hormone APIs and other complex HPAPIs. Many of these APIs are registered and used in originator finished dosage forms. Aspen Oss is also well known for its production of Heparin Sodium, Chorionic Gonadotrophin and several peptides.
Diosynth / Organon was acquired by Schering-Plough in 2007, followed by a merger of Schering-Plough and MSD (Merck in the US) in 2009. The API activities subsequently became part of the Aspen Group in 2013.
Fine Chemicals Corporation
South Africa
Fine Chemicals Corporation started in 1962 as a subsidiary of the South African Druggists Group (Ltd), the largest pharmaceutical company in Africa at the time.
Initial products were marketed domestically and included Narcotic APIs, Paracetamol, Ethoxyquin and non-nutritive sweeteners (cyclamates).
Fine Chemicals Corporation is one of a few Narcotic API manufacturers worldwide, recognized by the UN International Narcotics Board. This was granted per the 1960’s UN Convention and controls for the prevention of illicit manufacture and trade in narcotics.
Expansion into the International API market began in the mid-1970s, eventually leading to the discontinuation of high-volume commodity products.
In 2004, Fine Chemicals Corporation was acquired and became a wholly owned subsidiary of Aspen Pharmacare Holdings Limited.
Inspection overview
Seven successive US FDA inspections (last audit in July 2018) with no critical findings or form 483 issued. Manufacturing approval of the Japanese PMDA (last renewal in 2016). GMP certification by SAPHRA and by ANSM.